GATK Germline_SNP_INDEL_2.0 分析遗传病(耳聋)
一、本文是Gatk Germline spns-indels Pipeline 分析遗传病(耳聋)的升级版,目的是提供开箱即用的分析流程,尽可能简化部署和迁移。
更新内容如下:
-
人类参考基因组以及其他引用数据库文件版本由GRCh37(hg19)升级为GRCh38(hg38)
-
数据注释软件annovar更换为Ensemble vep(108.2),Annovar需要商业授权,vep为apache2.0 licence,可以随意使用
-
Pipeline用到的软件由预先安装改为docker+conda首次使用时安装,初次运行初始化环境下载必要文件,迁移更方便
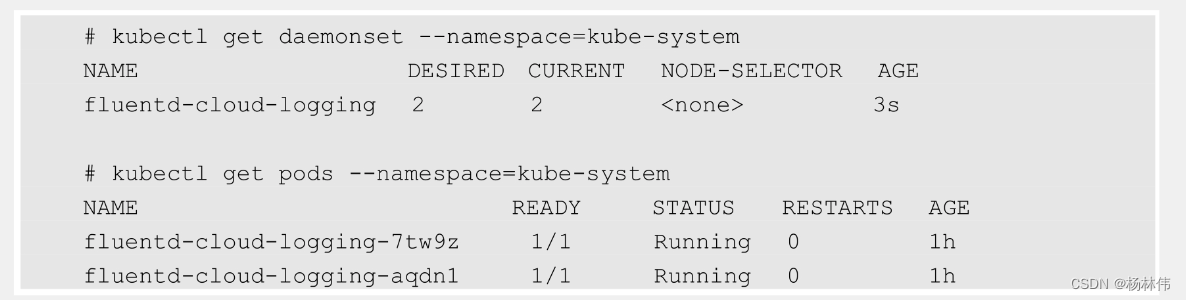
二、 流程概览图如下

| 流程输入 | SRR9993255_R1.fastq.gz SRR9993255_R2.fastq.gz 测试数据下载 [SRX6732499: Targeted NGS of human: blood sample 1 ILLUMINA (Illumina HiSeq X Ten) run: 679,472 spots, 203.8M bases, 80.8Mb downloads 如果下载数据是sra,可以用NCBI官方工具sra-toolkit拆分成fastq.gz文件 fastq-dump SRR9993255 --split-3 --gzip 得到SRR9993255_R1.fastq.gz SRR9993255_R2.fastq.gz 分析流程文件(可一键导入sliverworkspace运行)及报告文件,conda环境文件下载,导入操作 |
|---|---|
| 运行环境 | docker image based on ubuntu21.04 Conda Mamba(默认使用清华源) ssh |
| 分析软件 | - bwa=0.7.17 - sambamba=0.8.2 - samtools=1.16.1 - bedtools=2.30.0 - fastp=0.23.2 - gatk 4.3.0.0 - ensembl-vep=108.2 |
| 输出结果 | SRR9993255.result.qc.xls 数据质控结果 SRR9993255.result.variants.xls 突变结果文件 |
环境搭建: 为了快速完成环境搭建,节省95%以上时间。
本文使用docker + conda (mamba) 作为基础分析环境,镜像获取:docker/docker-compoes 的安装及镜像构建见《基于docker的生信基础环境镜像构建》,docker镜像基于ubuntu21.04构建,并安装有conda/mamba,ssh服务。并尝试初次运行时初始化安装所需软件下载所需文件(作为代价首次运行时间会较长,切需网络通畅),即实现自动初始化的分析流程。
备注:docker运行的操作系统,推荐为Linux,windows,macOS系统改下docker可能部分功能(网络)不能正常运行
# 拉取docker镜像
docker pull doujiangbaozi/sliverworkspace:latest
# 查看docker 镜像
docker images
基础环境配置,docker-compose.yml 配置文件,可以根据需要自行修改调整
version: "3"
services:
SarsCov2:
image: doujiangbaozi/sliverworkspace:latest
container_name: Germline
volumes:
- /media/sliver/Data/data:/opt/data:rw #挂载原始数据,放SC2目录下
- /media/sliver/Manufacture/GL2/envs:/root/mambaforge-pypy3/envs:rw #挂载envs conda环境目录
- /media/sliver/Manufacture/GL2/config:/opt/config:rw #挂载config conda配置文件目录
- /media/sliver/Manufacture/GL2/ref:/opt/ref:rw #挂载reference目录
- /media/sliver/Manufacture/GL2/result:/opt/result:rw #挂载中间文件和输出结果目录
ports:
- "9024:9024" #ssh连接端口可以按需修改
environment:
- TZ=Asia/Shanghai #设置时区
- PS=20191124 #修改默认ssh密码
- PT=2024 #修改默认ssh连接端口
基础环境运行
# docker-compose.yml 所在目录下运行
docker-compose up -d
# 或者
docker-compose up -d -f /路径/docker-compose.yaml
# 查看docker是否正常运行,docker-compose.yaml目录下运行
docker-compose ps
# 或者
docker ps
docker 容器使用,类似于登录远程服务器
# 登录docker,使用的是ssh服务,可以本地或者远程部署使用
ssh root@192.168.6.6 -p9024
# 看到如下,显示如下提示即正常登录
(base) root@SliverWorkstation:~#
三. 分析流程
-
变量设置:
#样本编号 export sn=SRR9993255 #数据输入目录 export data=/opt/data #数据输出、中间文件目录 export result=/opt/result #conda安装的环境目录 export envs=/root/mambaforge-pypy3/envs #vep cache 版本 export vep_version=108 #设置可用线程数 export threads=8 #与耳聋相关基因及突变文件 export whitelist=/opt/ref/whitelist.csv -
数据过滤:
#conda检测环境是否存在,首次运行不存在创建该环境并安装软件 if [ ! -d "${envs}/fastp" ]; then mamba env create -f /opt/config/fastp.yaml fi conda activate fastp mkdir -p ${result}/${sn}/clean mkdir -p ${result}/${sn}/qc #使用默认参数 fastp \ -w ${threads} \ -i ${data}/Germline/${sn}_R1.fastq.gz \ -I ${data}/Germline/${sn}_R2.fastq.gz \ -o ${result}/${sn}/clean/${sn}_fastp_R1.fastq.gz \ -O ${result}/${sn}/clean/${sn}_fastp_R2.fastq.gz \ -j ${result}/${sn}/qc/${sn}_fastp.json \ -h ${result}/${sn}/qc/${sn}_fastp.html conda deactivate -
数据比对、排序、标记重复;质控
#conda检测环境是否存在,首次运行不存在创建该环境并安装软件 if [ ! -d "${envs}/align" ]; then mamba env create -f /opt/config/align.yaml fi conda activate align #参考基因组文件如果不存在,去broadinstitute下载 if [ ! -f "/opt/ref/hg38/hg38.fasta" ]; then mkdir -p /opt/ref/hg38 aria2c ftp://gsapubftp-anonymous@ftp.broadinstitute.org/bundle/hg38/Homo_sapiens_assembly38.fasta.gz -d /opt/ref/hg38 -o hg38.fasta.gz cd /opt/ref/hg38 && gzip -d /opt/ref/hg38/hg38.fasta.gz #如果索引文件不存在,创建索引 if [ ! -f /opt/ref/hg38.fasta.amb ] || [ ! -f /opt/ref/hg38/hg38.fasta.ann ] || [ ! -f /opt/ref/hg38/hg38.fasta.bwt ] || [ ! -f /opt/ref/hg38/hg38.fasta.pac ] || [ ! -f /opt/ref/hg38/hg38.fasta.sa ]; then bwa index /opt/ref/hg38/hg38.fasta fi fi #如果samtools索引不存在,创建 if [ ! -f "/opt/ref/hg38/hg38.fasta.fai" ]; then samtools faidx /opt/ref/hg38/hg38.fasta fi cd ${result} mkdir -p ${result}/${sn}/align #数据比对、排序 bwa mem \ -t ${threads} -M \ -R "@RG\\tID:${sn}\\tLB:${sn}\\tPL:Illumina\\tPU:NextSeq550\\tSM:${sn}" \ /opt/ref/hg38/hg38.fasta \ ${result}/${sn}/clean/${sn}_fastp_R1.fastq.gz \ ${result}/${sn}/clean/${sn}_fastp_R2.fastq.gz \ | sambamba view -S -f bam -l 0 /dev/stdin \ | sambamba sort -t ${threads} -m 2G \ --tmpdir=${result}/${sn} \ -o ${result}/${sn}/align/${sn}_sorted.bam /dev/stdin ulimit -n 10240 #标记重复 sambamba markdup \ --tmpdir ${result}/${sn} \ -t ${threads} \ ${result}/${sn}/align/${sn}_sorted.bam \ ${result}/${sn}/align/${sn}_marked.bam mv ${result}/${sn}/align/${sn}_marked.bam.bai ${result}/${sn}/align/${sn}_marked.bai rm -f ${result}/${sn}/align/${sn}_sorted.bam #数据文件没有附带bed文件,这里用bedtools反向计算一个 bedtools genomecov -ibam ${result}/${sn}/align/${sn}_marked.bam -bg > ${result}/${sn}/align/${sn}_bedtools.bed bedtools merge -i ${result}/${sn}/align/${sn}_bedtools.bed > ${result}/${sn}/align/${sn}_merged.bed if [ ! -f "${envs}/bamdst/bamdst" ]; then apt-get update && apt-get install -y git make gcc zlib1g-dev git clone https://github.com/shiquan/bamdst.git "${envs}/bamdst" cd ${envs}/bamdst make cd ${result} fi ${envs}/bamdst/bamdst -p ${result}/${sn}/align/${sn}_merged.bed -o ${result}/${sn}/qc --cutoffdepth 20 ${sn}/align/${sn}_marked.bam & samtools flagstat --threads ${threads} ${result}/${sn}/align/${sn}_marked.bam > ${result}/${sn}/qc/${sn}_marked.flagstat & wait conda deactivate -
Gatk 获取碱基质量校准table
#conda检测环境是否存在,首次运行不存在创建该环境并安装软件 if [ ! -f "${envs}/gatk/bin/gatk" ]; then mkdir -p ${envs}/gatk/bin #替代下载地址 #https://github.com/broadinstitute/gatk/releases/download/4.3.0.0/gatk-4.3.0.0.zip aria2c https://download.yzuu.cf/broadinstitute/gatk/releases/download/4.3.0.0/gatk-4.3.0.0.zip -d ${envs}/gatk/bin -o gatk.zip apt-get install -y unzip cd ${envs}/gatk/bin unzip -o gatk.zip mv ${envs}/gatk/bin/gatk-4.3.0.0/* ${envs}/gatk/bin/ rm -rf ${envs}/gatk/bin/gatk-4.3.0.0 #chmod +x ${envs}/bin/gatk cd ${result} fi if [ ! -f "/opt/ref/hg38/dbsnp_146.hg38.vcf.gz" ]; then aria2c ftp://gsapubftp-anonymous@ftp.broadinstitute.org/bundle/hg38/dbsnp_146.hg38.vcf.gz -d /opt/ref/hg38 fi if [ ! -f "/opt/ref/hg38/dbsnp_146.hg38.vcf.gz.tbi" ]; then aria2c ftp://gsapubftp-anonymous@ftp.broadinstitute.org/bundle/hg38/dbsnp_146.hg38.vcf.gz.tbi -d /opt/ref/hg38 fi if [ ! -f "/opt/ref/hg38/Mills_and_1000G_gold_standard.indels.hg38.vcf.gz" ]; then aria2c ftp://gsapubftp-anonymous@ftp.broadinstitute.org/bundle/hg38/Mills_and_1000G_gold_standard.indels.hg38.vcf.gz -d /opt/ref/hg38 fi if [ ! -f "/opt/ref/hg38/Mills_and_1000G_gold_standard.indels.hg38.vcf.gz.tbi" ]; then aria2c ftp://gsapubftp-anonymous@ftp.broadinstitute.org/bundle/hg38/Mills_and_1000G_gold_standard.indels.hg38.vcf.gz.tbi -d /opt/ref/hg38 fi if [ ! -f "/opt/ref/hg38/1000G_phase1.snps.high_confidence.hg38.vcf.gz" ]; then aria2c ftp://gsapubftp-anonymous@ftp.broadinstitute.org/bundle/hg38/1000G_phase1.snps.high_confidence.hg38.vcf.gz -d /opt/ref/hg38 fi if [ ! -f "/opt/ref/hg38/1000G_phase1.snps.high_confidence.hg38.vcf.gz.tbi" ]; then aria2c ftp://gsapubftp-anonymous@ftp.broadinstitute.org/bundle/hg38/1000G_phase1.snps.high_confidence.hg38.vcf.gz.tbi -d /opt/ref/hg38 fi if [ ! -x "$(command -v python)" ]; then mamba env create -f ${envs}/gatk/bin/gatkcondaenv.yml fi if [ ! -x "$(command -v java)" ]; then mamba install -y openjdk=8.0.332 fi conda activate gatk if [ ! -f "/opt/ref/hg38/hg38.dict" ]; then gatk CreateSequenceDictionary -R /opt/ref/hg38/hg38.fasta -O /opt/ref/hg38/hg38.dict fi if [ ! -f "/opt/ref/hg38/hg38.exon.interval_list" ]; then gatk BedToIntervalList \ -I /opt/ref/hg38/hg38.exon.bed \ -SD /opt/ref/hg38/hg38.dict \ -O /opt/ref/hg38/hg38.exon.interval_list fi gatk BaseRecalibrator \ --known-sites /opt/ref/hg38/dbsnp_146.hg38.vcf.gz \ --known-sites /opt/ref/hg38/Mills_and_1000G_gold_standard.indels.hg38.vcf.gz \ --known-sites /opt/ref/hg38/1000G_phase1.snps.high_confidence.hg38.vcf.gz \ -L /opt/ref/hg38/hg38.exon.interval_list \ -R /opt/ref/hg38/hg38.fasta \ -I ${result}/${sn}/align/${sn}_marked.bam \ -O ${result}/${sn}/align/${sn}_recal.table conda deactivate -
Gatk 应用碱基校准、获取insert size 统计信息
conda activate gatk gatk ApplyBQSR \ --bqsr-recal-file ${result}/${sn}/align/${sn}_recal.table \ -L /opt/ref/hg38/hg38.exon.interval_list \ -R /opt/ref/hg38/hg38.fasta \ -I ${result}/${sn}/align/${sn}_marked.bam \ -O ${result}/${sn}/align/${sn}_bqsr.bam & gatk CollectInsertSizeMetrics \ -I ${result}/${sn}/align/${sn}_marked.bam \ -O ${result}/${sn}/qc/${sn}_insertsize_metrics.txt \ -H ${result}/${sn}/qc/${sn}_insertsize_histogram.pdf & wait conda deactivate -
Gatk HaplotypeCaller 获取snp/indel突变:
conda activate gatk mkdir -p ${result}/${sn}/vcf gatk HaplotypeCaller \ -R /opt/ref/hg38/hg38.fasta \ -L /opt/ref/hg38/hg38.exon.interval_list \ -I ${result}/${sn}/align/${sn}_bqsr.bam \ -D /opt/ref/hg38/dbsnp_146.hg38.vcf.gz \ -O ${result}/${sn}/vcf/${sn}.vcf conda deactivate -
单个样本数据不够运行VQSR,直接执行硬过滤,过滤参考数值见#https://gatk.broadinstitute.org/hc/en-us/articles/360035532412?id=11097
conda activate gatk
#https://gatk.broadinstitute.org/hc/en-us/articles/360035532412?id=11097
gatk VariantFiltration \
-R /opt/ref/hg38/hg38.fasta \
-V ${result}/${sn}/vcf/${sn}.vcf \
-O ${result}/${sn}/vcf/${sn}_snp.vcf \
--filter-name "SNP_DQ" \
--filter-expression "DQ < 2.0" \
--filter-name "SNP_MQ" \
--filter-expression "MQ <40.0" \
--filter-name "SNP_FS" \
--filter-expression "FS > 60.0" \
--filter-name "SNP_SOR" \
--filter-expression "SOR > 3.0" \
--filter-name "SNP_MQRankSum" \
--filter-expression "MQRankSum < -12.5" \
--filter-name "SNP_ReadPosRankSum" \
--filter-expression "ReadPosRankSum < -8.0"
gatk VariantFiltration \
-R /opt/ref/hg38/hg38.fasta \
-V ${result}/${sn}/vcf/${sn}_snp.vcf \
-O ${result}/${sn}/vcf/${sn}_filtered.vcf \
--filter-name "INDEL_DQ" \
--filter-expression "DQ < 2.0" \
--filter-name "INDEL_FS" \
--filter-expression "FS > 200.0" \
--filter-name "INDEL_SOR" \
--filter-expression "SOR > 10.0" \
--filter-name "INDEL_ReadPosRankSum" \
--filter-expression "ReadPosRankSum < -20.0" \
--filter-name "INDEL_InbreedingCoeff" \
--filter-expression "InbreedingCoeff < -0.8"
conda deactivate
- 使用vep注释突变结果:
#conda检测环境是否存在,首次运行不存在创建该环境并安装软件
if [ ! -d "${envs}/vep" ]; then
mamba env create -f /opt/config/vep.yaml
fi
mkdir -p /opt/result/${sn}/vcf
if [ ! -d "/opt/ref/vep-cache/homo_sapiens/${vep_version}_GRCh38" ]; then
aria2c https://ftp.ensembl.org/pub/release-${vep_version}/variation/indexed_vep_cache/homo_sapiens_vep_${vep_version}_GRCh38.tar.gz -d /opt/ref/
tar -zxvf /opt/ref/homo_sapiens_vep_${vep_version}_GRCh38.tar.gz -C /opt/ref/vep-cache/
fi
conda activate vep
vep \
-i /opt/result/${sn}/vcf/${sn}_filtered.vcf \
-o /opt/result/${sn}/vcf/${sn}_filtered_vep.xls \
--offline \
--cache \
--cache_version ${vep_version} \
--everything \
--dir_cache /opt/ref/vep-cache \
--dir_plugins /opt/ref/vep-cache/Plugins \
--species homo_sapiens \
--assembly GRCh38 \
--hgvs \
--fasta /opt/ref/hg38/hg38.fasta \
--force_overwrite \
--format vcf \
--tab \
--fork 8 \
--offline
conda deactivate
-
编写脚本匹配whitelist基因,突变过滤后vcf文件,vep注释后的文件,得到最终结果
#需借用gatk环境中的python来运行 conda activate gatk python ${envs}/GermlineVepAnnotationUtil.py \ --whitelist=${whitelist} \ -v ${result}/${sn}/vcf/${sn}_filtered.vcf \ -a ${result}/${sn}/vcf/${sn}_filtered_vep.xls \ -o ${result}/${sn}/${sn}.result.variants.xls conda deactivate -
编写脚本从fastp、bamdst、gatk CollectInsertSize 输出获取数据质控信息
conda activate gatk python ${envs}/GermlineQcUtil.py \ --out=/opt/result/${sn}/${sn}.result.qc.xls \ --sample-fastp=/opt/result/${sn}/qc/${sn}_fastp.json \ --sample-bamdst=/opt/result/${sn}/qc/coverage.report \ --sample-insertsize=/opt/result/${sn}/qc/${sn}_insertsize_metrics.txt conda deactivate -
结果确认,IGV bam文件和突变位置:

-
输出报告(报告模板见流程压缩包):

![[基因遗传算法]进阶之六:VRP的进阶经典问题的解码如何写](https://img-blog.csdnimg.cn/4c2d559c42764f39ba2105d3a21ca30d.png)