今日知识点:Co-ordinate bond (or Dative covalent bond)
例题
Q: When solid aluminium chloride is heated, Al2Cl6 is formed. Which bonding is present in Al2Cl6?
A Covalent and co-ordinate (dative covalent)
B Covalent only
C Ionic and co-ordinate (dative covalent)
D Ionic only
解析
Co-ordinate bond (also known as dative covalent bond): a covalent bond in which both electrons in the bond come from the same atom.
The structure of Al2Cl6 looks like the following:
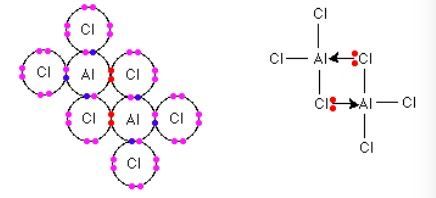
The electrons shared between Al and Cl both come from Cl (Marked in red). When drawing the Lewis structure, use arrows to point from the electron sufficient atom to the electron deficient atom.
Choose A
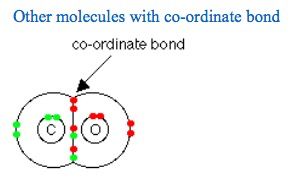

下面我们为大家准备了一道同类型的题目,请大家一起来试试解答。
What is the correct number of bonds of each type in the Al2Cl6 molecule?
covalent
co-ordinate
(dative covalent)
A
6
1
B
6
2
C
7
0
D
7
1
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
正确答案: B








![[附源码]计算机毕业设计springboot云南美食管理系统](https://img-blog.csdnimg.cn/7971d0b780fd403ab8e344b34c3aac60.png)