Ideas:
Carbon atoms that are bound to four different atoms or groups are said to be asymmetric (chiral)
The bonds formed by an asymmetric carbon can be arranged in two different mirror images (stereoisomers) of each other
Stereoisomers are either right-handed or left-handed and typically have completely different biological activities
Asymmetric carbons are key features of amino acids, carbohydrates, and nucleic acids
Biomolecules are stereospecific, because they also are chiral and their binding sites may be recognized by different enantiomers.
与四个不同原子或基团结合的碳原子被称为不对称的(手性的)
不对称碳形成的键可以排列成彼此的两个不同的镜像(立体异构体)
立体异构体有左旋和右旋两种,通常具有完全不同的生物活性
不对称碳是氨基酸、碳水化合物和核酸的主要特征
生物分子是立体特异性的,因为它们也是手性的,它们的结合位点可以被不同的对映体识别。
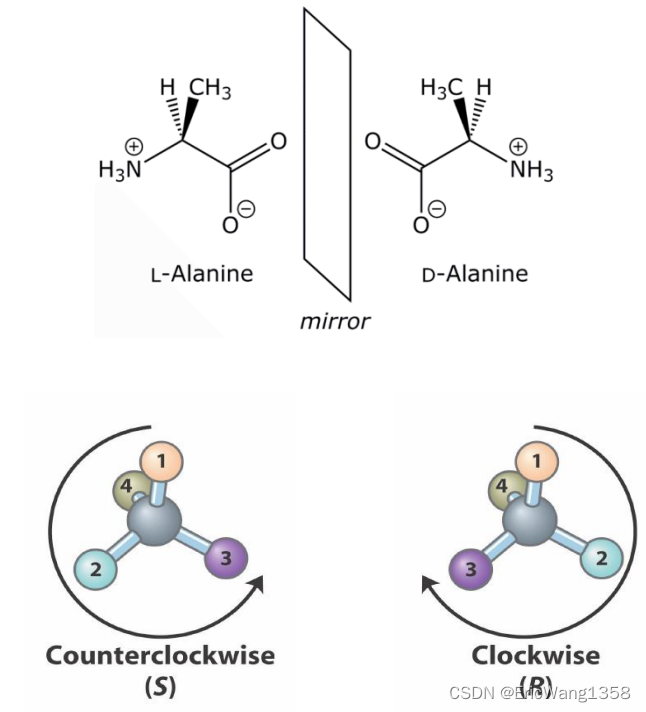
How?
• Locate the chiral center, identify its four substituents and assign priority from 1 (highest) to 4 (lowest) to each substituent.
• Orient the molecule so that the group of lowest priority (4) is directed away from you.
• Read the three groups projecting toward you in order from highest (1) to lowest priority (3).
• If the groups are read clockwise, the configuration is R (right); if they are read counterclockwise, the configuration is S (left).
定位手性中心,确定它的四个取代基,并为每个取代基分配从1(最高)到4(最低)的优先级。
定向分子,使最低优先级的基团(4)朝向远离你的方向。
按照最高优先级(1)到最低优先级(3)的顺序阅读向你投射的三组。
顺时针读,则构型为R(右);如果顺时针读取,则构型为S(左)。
How to assign the priority?
To locate the chiral center, identify its four substituents, and assign priority, you can follow the Cahn-Ingold-Prelog (CIP) rules, which are commonly used in organic chemistry. These rules are based on the atomic number of the atoms attached to the chiral center. Here's how to assign priority:
-
Identify the chiral center: Look for the carbon atom that is bonded to four different substituents. This carbon atom is the chiral center.
-
Assign priority based on atomic number: Examine the atoms directly bonded to the chiral center and assign priority based on their atomic numbers. The atom with the highest atomic number gets the highest priority (1), and the atom with the lowest atomic number gets the lowest priority (4).
-
If two substituents are the same, go one step further: Sometimes, two substituents may have the same atom directly bonded to the chiral center. In such cases, look at the atoms bonded to these substituents until you find the point of difference to determine their priority.
-
Priority order: Assign priorities based on the atomic numbers of the atoms bonded to the chiral center, following these rules:
- Priority 1: The atom with the highest atomic number.
- Priority 2: The atom with the second-highest atomic number.
- Priority 3: The atom with the third-highest atomic number.
- Priority 4: The atom with the lowest atomic number.
Once you have assigned priorities to all four substituents, you can use this priority order to determine the stereochemistry of the chiral center, such as whether it is R (clockwise) or S (counterclockwise) in terms of its configuration.
Keep in mind that sometimes, you may need to consider double bonds or other functional groups when determining the priorities, but the basic principle is to compare the atomic numbers of the directly bonded atoms.
要找到手性中心,识别其四个取代基,并分配优先级,您可以遵循有机化学中常用的Cahn-Ingold-Prelog(CIP)规则。这些规则基于连接到手性中心的原子的原子序数。以下是如何分配优先级的方法:
-
识别手性中心:查找与四个不同取代基连接的碳原子。这个碳原子就是手性中心。
-
基于原子序数分配优先级:检查直接连接到手性中心的原子,并根据它们的原子序数分配优先级。具有最高原子序数的原子获得最高优先级(1),而具有最低原子序数的原子获得最低优先级(4)。
-
如果两个取代基相同,请进一步检查:有时,两个取代基可能具有与手性中心直接连接的相同原子。在这种情况下,查看连接到这些取代基的原子,直到找到区分它们优先级的不同之处。
在分配手性中心的优先级时,如果存在双键或其他功能基团,您需要考虑这些功能基团的影响。以下是一些常见情况的一般指导原则:
-
双键:如果双键存在于取代基或它们直接连接的原子中,通常将双键上的原子视为连接两个相同原子的等效原子。例如,如果一个取代基有一个双键,而另一个取代基有一个氢原子,那么具有氢的取代基将具有较低的优先级。
-
氢原子:氢原子通常具有较低的优先级,因为它们的原子序数最低。然而,当所有其他原子相同时,氢原子可能不会影响优先级的分配。
-
具有相同原子的多个取代基:如果多个取代基具有相同的原子(例如,两个取代基都是氧原子),则必须继续查看连接到这些原子的其他原子,以区分它们的优先级。
-
功能基团:一些功能基团可能会影响优先级分配。例如,卤素原子(氟、氯、溴、碘)通常具有较高的原子序数,因此它们可能具有较高的优先级。羟基(-OH)和氨基(-NH2)等功能基团也可能影响优先级分配,具体情况取决于连接到它们的原子。
Molecular recognition is carried out by some special biomolecules (e.g. receptors and enzymes) that are themselves spatially arranged in a specific direction. Most biomolecules possess handedness and their binding sites can thus distinguish between the right-handed or left-handed compounds.
分子识别是由一些特殊的生物分子(例如受体和酶)执行的,它们本身以特定方向的空间排列。大多数生物分子具有手性,因此它们的结合位点可以区分右手或左手的化合物。